Abstract
Background: Worldwide, patients with newly diagnosed multiple myeloma (NDMM) undergoing upfront autologous stem cell transplantation (ASCT) have different characteristics and survival outcomes, likely due to variances in transplant activity, patient and health economic factors including access to new myeloma therapies. The goal of this retrospective study was to analyze differences in regional outcomes.
Methods: Data on patient characteristics and transplant outcomes for patients with NDMM who received an upfront ASCT between 2013 and 2017 were provided to the Worldwide Network for Blood and Marrow Transplantation (WBMT) by the European Society for Blood and Marrow Transplantation (EBMT), the Center for International Blood and Marrow Transplantation (CIBMTR), the Asian Pacific Blood and Marrow Transplant Group (APBMT), the Australia and New Zealand Transplant and Cellular Therapies Registry (ANZTCT), the Eastern Mediterranean Blood and Marrow Transplant Group (EMBMT), the Latin American Bone Marrow Transplant Group (LABMT), and the Ottawa Canadian Registry. There primary endpoints were overall (OS) and non-relapse mortality (NRM) and the secondary were progression free survival (PFS) and relapse incidence (RI). The Kaplan-Meier estimator and log-rank test were used for OS and PFS, and the crude cumulative incidence estimator and Gray's test were used for competing events (RI and NRM).
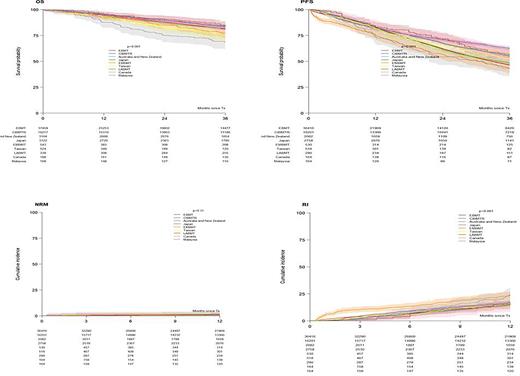
Results: 61725 patients from 629 were included: 37549 (61%) from EBMT, 16217 (26%) CIBMTR), 3815 (6%) APBMT (3122 Japan, 524 Taiwan and 169 Malaysia), 3,164 (5%) ANZTCT, 543 (0.9%) EMBMT, 339 (0.5%) LABMT and 188 (0.3%) Ottawa). The annual number of ASCTs steadily increased between 2013 (n=11317) and 2017 (n=13498) with the biggest relative increase seen in LABMT (from 36 to 109). Males comprised 58% and the median age at diagnosis was 59.9 years (IQR: 53.6-64.9). Race data was available in 45%: 73% White, 16% Asian and 11% African American. The predominant phenotypes were IgG (54%), light chain (24%) (lowest (4%) in Malaysia and highest (38%) in EMBMT), and IgA (19%) (lowest (13%) in EMBMT and highest (24%) in Ottawa). The ISS stage at diagnosis (54% available) was I in 38%, II 35% and III 27% (III lowest (24%) in ANZTCT and highest (45%) in LABMT). Cytogenetic risk (44% available) was standard in 70% and high in 30% (high risk was lowest (5%) in EMBMT, and highest (62%) in Ottawa). The median time from diagnosis to ASCT was 7 months (IQR:5.5-9.9) (shortest (6.4) in CIBMTR and Ottawa, and longest (13) in LABMT). The median age at ASCT was 60.8 years (IQR: 54.6-65.8) (lowest (53.6) in EMBMT, and highest (62) in Ottawa). Only 5% of patients were older than 70 years (lowest (3.5%) in EBMT, and highest (9.8%) in CIBMTR). HCT-CI at ASCT (28% missing) was reported as low risk (0) in 52%, intermediate (1-2) 25% and high risk (≥3) 23% (high risk was lowest (5.5%) in LABMT and highest (42%) in CIBMTR). The Karnofsky score at ASCT (2.2 % missing) was 100 in 40% and ≤90 in 60% (≤90 was lowest (44%) in LABMT and highest (92%) in Ottawa). Disease status (9.7% missing) was CR in 19%, VGPR 38%, PR 36%, MR/SD 5% and refractory 2%. A ≥VGPR status at ASCT was 60% in EBMT, 55% CIBMTR, 39% ANZTCT, 51% Japan, 64% EMBMT, 71% Taiwan, 76% LABMT, 51% Ottawa and 54% Malaysia. The most frequent preparative regimen was melphalan 200 mg/m2 (82 %) (lowest (60%) in Malaysia and highest (90%) in Ottawa), 140 mg/m2 accounted for 14% and others for 4%. Tandem ASCT was reported in 6.7% (10% in EBMT, 1.3% in CIBMTR and 0.6% LABMT and Taiwan). The source of stem cells was peripheral blood in 99.8%. Of the 11% reported with post-ASCT maintenance treatment, 51% received lenalidomide. The median follow-up was 41.1 months (95% CI: 40.5-41.6, IQR:19.2-60.4). Regionally, at 3 years, the OS was : 81%, 84%, 82%, 83%, 77%, 75%, 84%, 82%, 69% (p<0.001), the PFS was 46%, 55%, 48%, 63%, 44%, 53%, 56%, 53%, 43% (p<0.001) (Figure) and the RI was:16%, 15%, 16%, 17%, 24%, 16%, 16%, 13%, 24% (p<0.001) for EBMT, CIBMTR, ANZTCT, Japan, EMBMT, Taiwan, LABMT, Ottawa and Malaysia, respectively. NRM at 1 year was between 1-2% in each registry (Figure).
Conclusions: This large worldwide study of patients with NDMM treated with upfront ASCT revealed marked regional differences in transplant activity and patient characteristics. NRM was low worldwide but with differences in relapse incidence and survival potentially reflect variable health economic factors, including different access to new myeloma drugs.
Disclosures
Garderet:BMS: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. Hari:BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; AbbVie: Honoraria; Pharmacyclics: Consultancy; Millennium: Research Funding; Iovance: Current Employment; Spectrum Pharmaceuticals: Research Funding; Novartis: Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Incyte: Honoraria; Kite: Consultancy, Honoraria; GlaxoSmithKline: Honoraria. Cowan:Abbvie: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy; BMS: Consultancy, Research Funding; EUSA: Consultancy; GSK: Consultancy; Harpoon: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Research Funding; Sanofi: Research Funding; Secura Bio: Consultancy. Takamatsu:SRL: Consultancy; Janssen: Honoraria; Ono: Honoraria; Sanofi: Honoraria; Bristol-Myers Squibb: Honoraria. Hamad:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mian:Sanofi: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. McCurdy:Sanofi: Honoraria; GSK: Honoraria; Amgen: Honoraria; Forus: Honoraria; BMS: Honoraria, Research Funding; Janssen: Honoraria; Takeda: Honoraria. Snowden:Novartis: Speakers Bureau; Mallinckrodt: Speakers Bureau; Gilead: Speakers Bureau; Janssen and Jazz: Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees; Kiadis: Other: clinical trial IDMC membership . Schönland:Pfizer: Honoraria; Takeda: Honoraria, Other: Travel Support; Janssen: Honoraria, Other: travel support, Research Funding; Prothena: Honoraria, Other: Travel Support, Research Funding. McLornan:ABBVIE: Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau; JAZZ: Honoraria, Speakers Bureau; NOVARTIS: Honoraria, Research Funding, Speakers Bureau. Hayden:Amgen: Other: Participation in Advisory Board. Sureda:TAKEDA: Consultancy, Honoraria, Speakers Bureau; MSD: Honoraria; SANOFI: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; ROCHE: Consultancy, Honoraria; BMS: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; GILEAD: Consultancy. Greinix:Gilead, Novartis, Sanofi, Cellgene: Consultancy; Amgen, Gilead, Novartis, Sanofi, Takeda, Therakos: Speakers Bureau. Atsuta:Novartis Pharma KK: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; Astellas Pharma Inc.: Honoraria; AbbVie GK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal